SUMMARY
Presentation and explanation of the thermodynamic principle
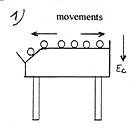
It is a new heat-pump process which consists in transferring heat by a gas between two close plates thanks to an electric field created at the surface of the warm plate called “electrostatic” which polarizes and attracts the gas molecules on this plate in order to make them yield energy by thermal accommodation ( (1) diagram below). The molecules behave as if they were in contact with a colder plate ( (2) diagram below).
Thermodynamic cycle
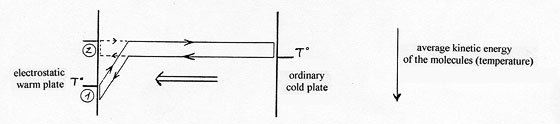
The heat transfer occurs from the ordinary cold plate towards the warm electrostatic plate,
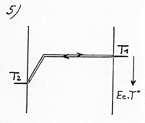
within the limits of a difference of temperature corresponding to the acceleration of the molecules in the attraction field (diagram 5).
is determined by the gradient of temperature in the part of the gas which is not submitted to the electric field, that is to say nearly the whole volume of the gas. So we’ll adopt the formula giving the heat current by thermal induction in a gas between two plates with two different temperatures, with a modification for the temperature of the electrostatic plate T2’.
|
Q = |
l |
T1-T2’ |
|
|
|
d + 2g |
with l = thermal conductivity of the gas
d = distance between the plates.
g = 2.7 fmp (free mean path of the molecules in the gas).
Consequently, the rarefaction of the gas doesn’t attenuate the power of thermal transfer
compared
to a gas being at the atmospheric pressure, as long as the free mean path
of the molecules doesn’t equal the distance between the plates. With plates
distant of 0.1mm, the transfer power is only reduced of 20% for a pressure
of 10-2 bar, 75% for 10-3 bar.
In a gas submitted to a field of attraction such as gravity, there is a variation of pressure but also of temperature, the gradient of which being parallel to the field of attraction.
So, in the atmosphere, the temperatures cools down as we rise in altitude.
Consequently, if we put two metal plates in thermal contact and in between a thin horizontal layer of gas in which reigns a very dense field of attraction, then a difference of temperature is going to take place between the two plates. The upper plate will yield heat and will get cooler while the lower plate will get warmer by absorbing this heat. The heat will flow spontaneously ( without work) from one plate to the other through the gas by thermal conduction.
The thermodynamic principle of the pump.
It consists in transferring heat, through a gas between two plates with different temperatures, from the cold plate towards the warm plate thanks to an electric field created at the surface of the warm plate called “electrostatic” which attracts the gas molecules to make them yield some of their energy by thermal accommodation. By penetrating into the electric field, each molecule is attracted and its kinetic energy increases, which results in an increase of the temperature of the plate in contact with the warm molecules:
The kinetic energy of the molecules Ek is linked to the temperature T :
Ek= 3/2 KT ( for a monatomic gas or a rare gas).
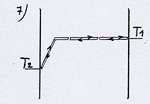
A vivid way of imagining this phenomenon is to replace gas molecules by a multitude of billiard balls in motion on a table. This table has a gap on one side (see diagram 1).
|
|
A vivid way of imagining this phenomenon is to replace gas molecules by a multitude of billiard balls in motion on a table. This table has a gap on one side (see diagram 1). For the imagery to be right, one has to disregard the loss of energy which tends to reduce the motion, such as the slight friction of the balls on the billiard cloth as well as the loss of energy due to the collisions ( the balls get slightly warmer when they collide hence a global loss of kinetic energy after a collision). |
One could also note that the average kinetic energy of the balls which hit the left side of the table, at the bottom of the gap, is superior to the average kinetic energy of the balls on the horizontal part of the table.
Each ball that rolls down the gap has its kinetic energy which increases with a value corresponding to the potential energy of the ball falling in the gap. As the average kinetic energy is equivalent to the temperature, the temperature of the balls hitting the left side of the table is superior to the temperature of the balls hitting the right side.
NB: In accordance with this representation, it seemed judicious to me to increase the temperature downwards on all the diagrams. A lower scale on the diagram corresponding to a higher temperature.
The thermal accommodation principle.
Thermal transfer between two ordinary plates with different temperatures.
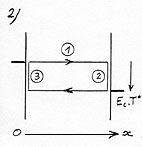
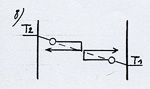
In order to make the representation simpler, let’s imagine two plates close the one from the other with different temperature and some rarefied gas in between so that the molecules can oscillate between the two plates without colliding with other molecules.
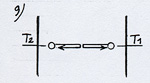
Let’s first observe one of them when it goes towards the right- hand-side plate (see (1) diagram 2)(The arrowed cycle indicates the direction the molecule follows and its energetic level according to its position on the Ox axis between the plates. A horizontal line shows that the molecule moves with a constant velocity and energy between the plates.)
|
|
When it reaches the right hand side plate, which is warmer, the molecule adapts its kinetic energy to the temperature of the plate. It is going to get warmer by bouncing off the surface of the plate with a higher velocity after having drawn energy in the form of heat from the plate (see (2) diagram 2). This phenomenon is known under the name of thermal accommodation (or adaptation). |
More precisely, when a molecule at a temperature Ti hits a surface at a temperature Ts, there is exchange of energy and the molecule is re-emitted at an intermediate temperature Tr. A coefficient of accommodation is thus determined, which is the ratio of the average amount of energy effectively exchanged to the energy which could be exchanged if the re-emitted particles reached a perfect thermal equilibrium with the wall.
|
a = |
Tr - Ti |
|
Ts - Ti |
“a” is usually included between 0.4 and 0.85.
The coefficient “a” mainly depends on the nature of the gas. It is high with a gas with a high molecular mass (which is the case with this invention. Ref: Techniques de l’ingénieur B4020).
The opposite effect will occur when in contact with the left plate which is colder as the molecule will bounce off at lower velocity after having yielded energy in the shape of heat to the plate (see (3), diagram 2). This exchange multiplied by the many “round-trips”, since the average velocity of a gas molecule is about 400 to 500 m/s, added to the high density of the molecules contained in a gas, has the effect of progressively leveling the temperature of the two plates.
Influence of
the gaseous pressure on the power of heat transfer.
With a pressure (or density) corresponding to an average mean free path (mfp) superior to the distance between the plates, i.e. a rarefied gas, a slight variation of pressure will produce a variation in the number of those “energy carriers” that are the molecules and consequently a variation of the power of heat transfer.
|
|
For a pressure corresponding to an average mean free path inferior to the distance between the plates, i.e. a high density gas, some collisions will occur in the gas and this all the more as the density is high ( the mean free path in an argon gas at the atmospheric pressure is 50 nanometers (10-9m) or 0.05 micron). As can be seen on the diagram opposite, those collisions shorten the molecular thermodynamic cycles (1) but they also tend to level the average energy of the molecules after the rebound (2) which diminishes the difference of average kinetic energy on a given |
plane between O and x in the two moving directions of the molecules. The frequency of the energy exchanged against the walls always increases proportionally to the pressure. But this increase in the frequency is compensated by a decrease of the energy exchanged with each collision. If we take up the imagery of the “energy carriers” again, the number of carriers continually increases with the pressure, but when the free mean path is shorter than the distance between the plates, this increase is compensated by a diminution of their charge, hence a constant discharge of energy. The power of heat transfer doesn’t fluctuate with the pressure anymore, what confirms the following formula:
|
Q = |
l |
T1 – T2 |
|
d + 2g |
l = Thermal conductivity of the gas ; T1-T2 = temperature of the plates; d = distance between the two plates; g = 2.7 mfp (mean free path) for the heavy gas.
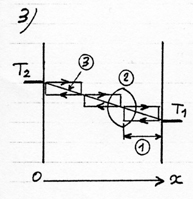
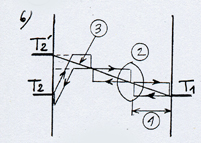
We can see in this example that the temperature in the gas would vary according to the position on the axis Ox between the plates, according to a gradient or a thermal slope ( (3) diagram 3). The symbolical representation of three cycles mainly means that the temperature of the gas, as in a rarefied gas, is linked to the moving direction of the molecules. But it would be more accurate to represent the temperature according to the moving direction and to draw two lines parallel to the thermal slope because the collisions occur at every level on the axis Ox. The division into three juxtaposed cycles, suggests that in a high density gas, everything takes place as if there were walls or extremely thin films set parallel to the plates, the mean free path corresponding to the distance between those walls.
Introduction of an attraction field
|
|
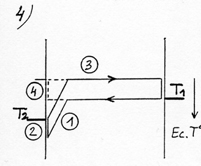
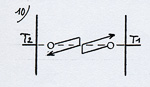
To make the demonstration simpler, we’ll use the rarefied gas again. Let’s suppose that the molecule when coming close to the left plate, called electrostatic, penetrates into an electric attraction field. This will be transcribed on the thermo-energetic graph with a gap ( (1) diagram opposite) similar to the billiard table represented before. |
This attraction field increases the kinetic energy of the molecule and is going to bring it to a temperature superior to the plate’s (with the limits of a difference of temperature between the plates corresponding to the potential energy of the attraction field). By thermal accommodation (or adaptation), it is going to yield energy when in contact with the plate (2) and set off again at a lower velocity. To set off again, the molecule has to “go up” the attraction field and loose as much kinetic energy as it had previously gained by “falling down”. The final result stands in a cooling down of the molecule (3), although originally, before penetrating into the attraction field, it was colder than the plate it was about to meet. This cooling down will be identical to the one it would have undergone on an ordinary plate with an identical difference of temperature between the molecule and the plate at the moment of the contact (4).
NB: It is now necessary to note that the potential energy of the attraction field only being equivalent to a small fraction of the average kinetic energy of the molecules ( a few dozens of degrees at the most, compared to the ambient temperature which is about 300 Kelvins), it is very unlikely for them to get trapped and they don’t need to recover thermal energy on the plate to get out of the attraction field which of course would annihilate the expected effect. This can be compared to a car driving over a pothole. It is only slightly slowed down and doesn’t need the power of its engine to get out of it.
|
|
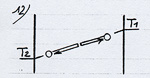
The energetic exchange described before tends to warm up the electrostatic plate and to cool the ordinary plate until the difference of temperature between the plates reaches a value corresponding to the potential energy of the attraction field. The thermodynamic equilibrium is reached when the average energy of the molecule in contact with each plate corresponds to the respective temperature of the plates (see diagram opposite). In this case there is globally no more heat transfer. This situation corresponds to the configuration “billiard table” on which the balls bounce off the walls while preserving their kinetic energy. With a stronger pressure (or a gas with a higher density) the fundamental principle remains the same, except that the molecules, once out of the attraction field and cooled, take up energy again by means of intermolecular collisions rather than on the cold plate. Out of the attraction field the energetic exchange process remains identical to the one between two ordinary plates with a temperature varying on the axis Ox according to a gradient or a thermal slope (see (3) on the diagram opposite) which besides determines the power of thermal transfer. So, when the thermal slope in this area is flat, the thermodynamic equilibrium is reached and the transfer power is cancelled (diagram 7).According to this diagram, the gas reigning in the attraction field ( against the electrostatic plate) would be in thermodynamic equilibrium in spite of the variation of temperature and in spite of the intermolecular collisions in this area. To be convinced of this, it is necessary to examine the conditions in which is equilibrium is established. As we have seen on the diagrams 3 and 6 the collisions tend to equalize the average energy of the two directions of motion of the molecules. If one consider, at a given time, the whole lot of molecules being on a plane parallel to the plates, included between O and x, the molecules moving leftward have an average energy higher than those moving rightward. But this difference tends to cancel itself for the molecules which have just collided (diagram 8). The thermodynamic equilibrium is reached when the molecules have an identical average energy in both directions of motion, that is to say when there is no more global exchange of energy in the collisions. In that case, the whole gas is at a uniform temperature, the average energy of the molecules is the same everywhere (diagram 9). If we now introduce an attraction field, the molecules move with a variable energy and not constant anymore as in the preceding cases ( which is represented by the sloping arrows). In the case which is represented diagram 10, where the temperature is uniform in the gas, the molecules gain or lose energy on their way and consequently, they meet a different energy, hence a global exchange of energy in the collisions. The gas isn’t in thermodynamic equilibrium. To take up the example of the diagram 3, the thermal exchange between the two plates would happen as is represented on the diagram 11. The thermodynamic equilibrium is reached when the molecules moving on each side have an identical average energy when the shock occurs. In that case there isn’t any global exchange of energy in the collisions anymore, although the temperature isn’t uniform in the gas (diagram 12). We have a natural example of a gas in thermodynamic equilibrium although its temperature isn’t uniform; this gas is the atmosphere in which the temperature and the pressure decrease when we rise in altitude: about 6°C per 1000 m. It is the attraction field of gravity which is at the origin of this variation of temperature and pressure. The masses of air compress and get warmer when they go down or release their pressure and cool down when they go up. The principle of this invention consists in creating a very powerful gravitational field in order to get a sufficient difference of temperature on a very short distance and one can consider that the warm electrostatic plate is the low altitude plate while the cold ordinary plate is the high altitude plate. |
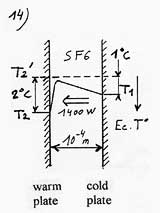
To calculate the power of thermal transfer (heat current) we will use the formula used with two ordinary plates with an adaptation for the temperature of the electrostatic plate. It isn’t the real temperature of the electrostatic plate that one must consider but the temperature beyond the attraction field ( T2’. See diagram 6), which with T1, defines the gradient of temperature in the gaseous space which is out of the attraction field. T2’ is equal to the real temperature of the plate (T2), to which one subtracts the difference of temperature corresponding to the potential energy of the attraction field.
|
Q = |
l |
T1 – T2’ |
|
d + 2g |
|
|
Let’s imagine the case of two plates separated of 0.1mm (10-4 m) and an attraction field equivalent to 2°C ( the example is voluntarily modest to better show the potentiality of this process). In thermal equilibrium, that is to say when the thermal slope is flat, out of the attraction field ( T1- T2’ = 0), the electrostatic plate would be warmer than the ordinary plate of 2°C. By reducing this gap of 1°C, one redresses the thermal slope of 1°C so, T1-T2’ = 1°C If the gas used is some SF6, the thermal conductivity of which is l = 0.14 w/m.K and if we disregard 2g if g is small compared to d, the heat current per square meter would be:
With a distance between the plates of 0.01 mm (10 microns) wich is concevable, and an attraction field equivalent to 20°C, we could get 140 KW/m² ! |