Explanation of the electrostatic principle.
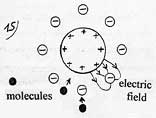
The attraction of molecules by polarization is known in the phenomenon of electrostriction. It is also at the origin of the formation of rare gas hydrates ( Arn H2O). In this example, the argon atoms get polarized and are attracted when close from the water molecule dipole.
The energy of acceleration of a molecule
penetrating in the electric field is given by:
E pol = ˝ a E2. With a = polarizability of the molecule and E = electric field.
So it is only the average value of the electric field in contact with the electrostatic plate which determines the acceleration energy of the molecules. A grid gives the acceleration energy of a molecule for a few gas and the corresponding heating.
Electrostatic
principle
|
|
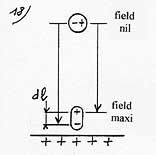
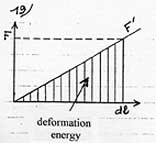
Yet, some of this energy is used to deform the molecule in the same way as energy is needed to set a spring in motion. As the force of deformation F’ varies linearly with dl (diagram 19), the average force of deformation is thus 1/2F, and the energy of deformation 1/2F.dl. Consequently, the remaining energy of 1/2Fdl is the attraction energy of the molecule on the plate. The polarizability of the molecule is µ = Q.dl/E (en Cm/Vm-1) So Q.dl = µ.E On the other hand the electrostatic force which separates the charges F = Q.E so F.dl = Q.E.dl = µ.E˛ |
Energy of attraction = ˝ F.dl = ˝ µ.E˛
This formula can be found in different books by different authors.
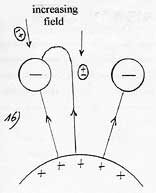
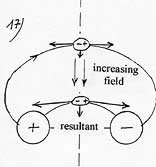
To put it differently the energy of attraction of a polarizable molecule only depends on the value of the electric field at the surface of the plate and this independently of the length of attraction L, that is to say the thickness of the zone of variable field to cross and also independently of the value of the gradient of the electric field. Indeed, for a same electric field of surface, if one doubles the thickness of the zone of variable field, one doubles the length of attraction L but at the same time, one reduces of a half the gradient of the electric field and hence, the gravity. Consequently, the attraction work remains identical.
The grid below gives the attraction energy (or attraction work) and the correspondence in heating degrees for a few examples of gas molecules penetrating in a field of 5 x 108 V/m ( 500 KV/mm) assessing they come from a zone where the field is nil.
The heating of 1°C is equal to about 2.10-23 Joule per molecule of monatomic gas, that is to say 3/2K, with K=1.38 x 10-23 Joule. For a polyatomic molecule such as SF6, the translation energy of the molecule now represents the half of its total energy, the other half being rotation-on-itself energy. To estimate correctly the heating of such a molecule, considering that only the translation energy increases when the molecule penetrates the electric field, one would have to divide by two the heating that one would have if it was a monatomic molecule (which only possesses the translation energy).
|
Gas |
Polarizability |
Attraction energy |
Degrees of heating |
|
Argon |
1.85 x 10-40 |
2 x 10-23
Joules |
1 Kelvin |