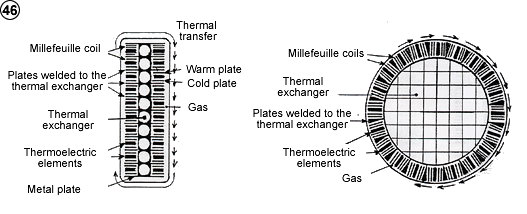
are first of all those of the actual heat pump, with an interest highly increased by the high coefficient of performance. Some other specific applications come from this high coefficient of performance, all these inventions coming to graft themselves on the basic process. It would become possible to cool the environment, for instance the water of a stream or a river, to deliver work, for instance electricity or to cool sea water to produce both electricity and fresh water. It becomes also possible to consider thermoelectric generators which could advantageously replace the actual generators, as well as the generating units and even the batteries for particular use.
but whose interest would be enlarged by a high C.O.P.
- Heating and air-conditioning of council estates or flats, hospitals, hotels, schools, auditoriums and offices.
- Water heaters, heating for swimming pools, greenhouses, soil for mushrooms culture, water for fish breeding.
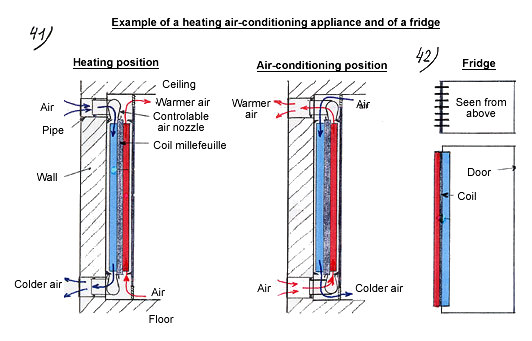
- Fridges (see diagram below).
- Refrigerators for preserved food and food industry, for the air-conditioning of port installations.
Heat recycling devices ( without external input of heat at the cold source)
- Fur, meat, wood, paper, cereals (corn, barley) drying.
- Evaporation for the concentration of aqueous solutions in food industry, paper manufacturing and chemical industry.
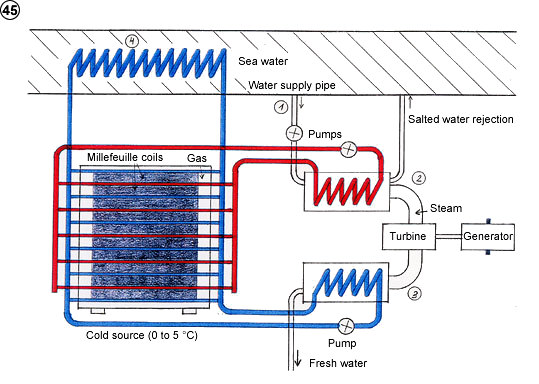
- To remove salt from the sea.
Provided that the process be able to deliver a warm source up to 120°C
One can note a potential market in:
- Oil refining
- Paper making
- Food industry
- Alcohol distilling
Other applications linked to compactness, simplicity, absence of noise.
- Small portable fridges
- Air conditioned containers
- Heating / air-conditioning appliance for individual lodging (diagram below).
Example of a heating – air conditioning appliance and of a fridge
|
|
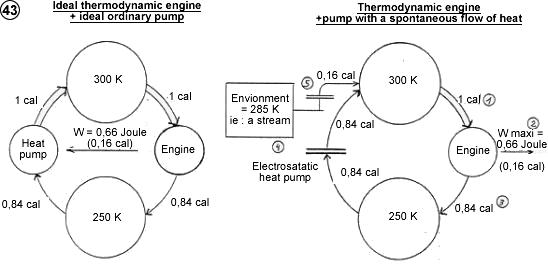
1) Conversion-of-ambient-temperature-into-work Station (Thermodynamic station with a sole thermal source)
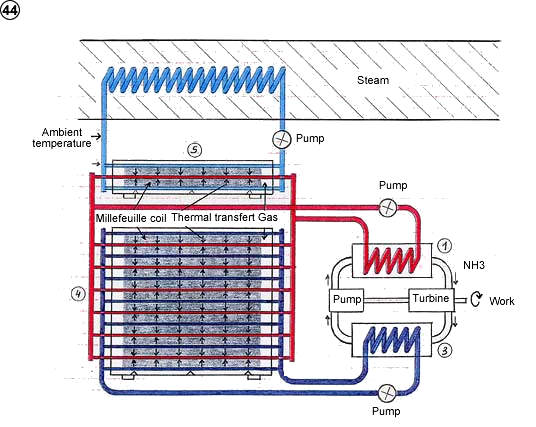
2) House supplying power and fresh water with sea water
3) Thermoelectric generator